SUMMARY
In June 2021, the US Patent and Trademark Office (USPTO) published an update to its study of America Invents Act (AIA) trials involving challenges to Orange Book-listed and biologic patents from September 16, 2012, through June 30, 2021.1 Here, we review the statistics and then give an overview of notable biologics decisions to date. With the number of petitions aimed at biologics patents growing from 8 petitions filed in FY2020 to at least 23 in FY2021, a more complete picture of how successful PTAB proceedings are in the biologics space will soon come into focus.
STATISTICS
Since post-grant proceedings became available, only 4% of all AIA petitions challenge Orange Book patents, and only 2% of petitions challenge biologic patents.2 The number of AIA challenges to Orange Book patents has decreased every year since 2015.3 Similarly, the number of biologic patent challenges have decreased year over year from 2017 to 2020.4 2021 was the first year to see a rise in the number of challenges to biologic patents since 2017.
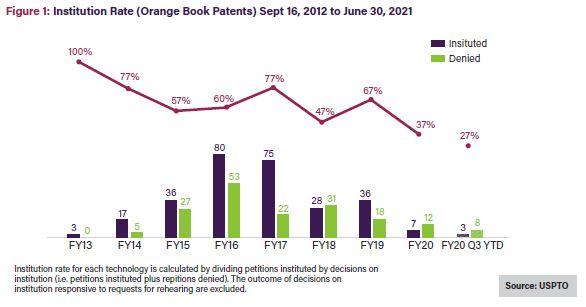
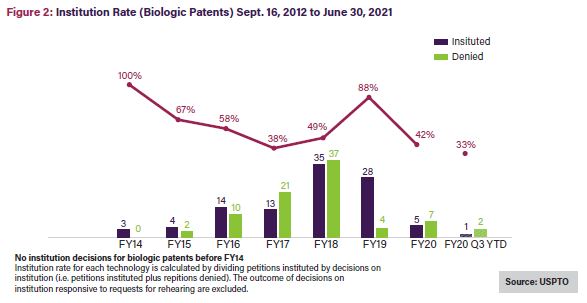
The all-time institution rate for Orange Book patents since 2012 averages 62%—close to the average across all technologies—while petitions challenging biologics have a 55% all-time average institution rate,5 the lowest rate for all utility patents.6 Noticeably, though, the institution rate for Orange Book patents, shown in Figure 1 below, has been on a generally downward trajectory over time, averaging 37% in 2020 and 27% in 2021.7 Challenges to biologic patents have seen a similar decline, with the institution rate falling by more than half from FY2019 to FY20208 and in 2021, to its lowest rate to date: 33%.9See Figure 2.
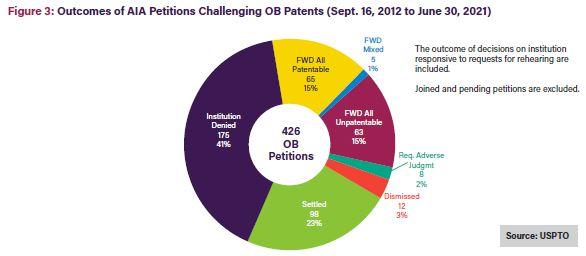
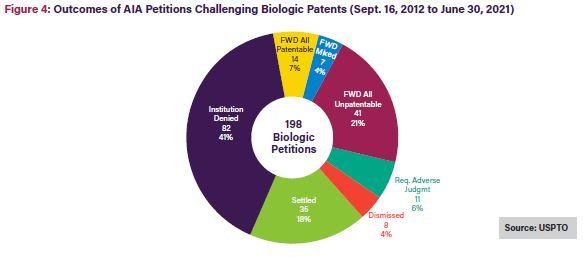
As shown in Figures 3 and 4 below, only 15% of Orange Book patent challenges result in final written decisions finding all claims unpatentable10; 21% of biologic patent challenges result in final written decisions finding all claims unpatentable.11 On a claim-by-claim basis, 31% of instituted Orange Book patent claims were found unpatentable, while a much larger 59% of instituted biologics claims were found unpatentable.12
As shown in the figures below, once instituted, biologic patent challenges are significantly more likely to result in final written decisions finding all claims unpatentable than challenges to Orange Book patents. This outcome disparity can be explained by the larger variety of claims covered by biologic patents, including claims to manufacturing methods, processes, and metabolites, which may be easier to challenge at the USPTO Patent Trial and Appeal Board (PTAB). In contrast, Orange Book patents only cover compound, formulation, and method claims.
Orange book listed patents typically come with Hatch-Waxman litigation, which factors significantly into the strategy of when, or even whether to challenge Orange Book patents at the PTAB. Some stakeholders find that there is less benefit to be realized from a win at the PTAB against an Orange Book patent due to the automatic 30-month stay of approval under the Hatch-Waxman framework. Others continue to find value in bringing PTAB challenges, for example, to put pressure on patent owners to settle, to clear out patents that are easier to challenge on a shorter 18-month timeline, or to get parallel district court litigation stayed pending the outcome of the PTAB challenge.
As for biologics patents, a relatively small number of biologics have been at the stage where they face potential competition from biosimilars in court (for example, only eight molecules have been involved in BPCIA litigation so far). That said, not every biologic requires waiting until a biosimilar challenge is ripe before approaching the PTAB. In fact, biosimilar makers seem to be filing challenges at the PTAB even before seeking FDA approval for their biosimilar products. Thus, biosimilar makers could be filing at the PTAB rather than engaging in the patent dance altogether. Based on the significant rise in biologic patent challenges in 2021 compared to filings from 2017-2020, the statistics seem to support this strategy as a method for biosimilar makers to clear out biologic patents standing in the way of FDA approval. In addition, with the approval of more biologics in the coming years, biosimilar makers could find value in filing PGRs due to the availability of additional Section 112 grounds that can be included in these proceedings.
To the extent that anything concrete can be taken from the USPTO’s statistics, it is that the biologic patent cancellation rate could be affecting the number of filings at the PTAB as reflected by the substantial increase in the number of filings in 2021. Some notable biologic post-grant proceedings are discussed next.
PTAB BIOLOGICS PROCEEDINGS
A. Rituximab (RITUXAN)
Since 2015, there have been 27 inter partes reviews (IPR) filed challenging 10 different patents covering rituximab. Out of the 10 patents challenged across the 27 proceedings, only two have been found to be unpatentable: U.S. Patent Nos. 8,821,873 and 9,296,821. Two other IPRs both challenging U.S. Patent No. 7,820,161, while instituted, ultimately failed to prove that the claims were unpatentable. All other IPRs were either terminated because of settlement or denied institution.
The ’873 patent is directed to a method of treating a patient with diffuse, large-cell lymphoma by administering anti-CD20 antibodies (e.g. Rituximab) in combination with stem cell transplantation. Pfizer filed a petition challenging all claims of the ’873 patent as obvious in two grounds over multiple prior art references.13 The PTAB consolidated the two grounds into one and ultimately found that the prior art provided a motivation for combining rituximab with stem cell transplantation and supported a POSA’s reasonable expectation of success in this combination treatment. Accordingly, the PTAB found all challenged claims unpatentable as obvious.14
The ’821 patent covers methods for treating low-grade or follicular Non-Hodgkin’s lymphoma by administering an effective amount of rituximab during a chemotherapeutic regimen. Celltrion filed a petition challenging all claims of the ’821 patent.15 After instituting on all grounds in view of SAS Inst., Inc. v. Iancu, 138 S.Ct. 1348 (2018), the PTAB held a subset of claims unpatentable as obvious, and the others anticipated.16
The ’161 patent covers a method of treating rheumatoid arthritis in a human by administering more than one intravenous dose of a therapeutically effective amount of rituximab and methotrexate. Celltrion filed a petition, with Pfizer joining, challenging all claims of the ’161 patent in three separate obviousness grounds.17 Because Celltrion did not put forth separate arguments with respect to references in two of the other grounds, the PTAB combined the references and instituted review on a single obviousness ground based on four references, one of which was the Rituxan Label.18 Biogen and Genentech (Patent Owners) challenged the admissibility of the Rituxan Label as prior art by arguing that the copyright date and the presence of the label on the FDA website did not establish that the label was publicly accessible. The PTAB agreed. Celltrion separately submitted a webpage copy of the Rituxan label along with a declaration from the Office Manager from Internet Archives explaining that the webpage copy of the label was a true and accurate copy of the printout. However, the PTAB held that Celltrion had not shown that the Rituxan Label was broadly disseminated and publicly accessible or that persons interested and ordinarily skilled in treating rheumatoid arthritis would have identified and visited Genentech’s website before the critical date. The PTAB then analyzed the instituted ground without reference to the Rituxan Label and held that the remaining references did not teach several limitations. As such, the PTAB confirmed the challenged claims.
Genentech has also asserted its patents related to Rituxan in four BPCIA litigations, all of which have settled or been voluntarily dismissed.
B. Trastuzumab (HERCEPTIN)
Since 2014, seven petitioners have filed 36 IPRs challenging 12 different patents, with mixed results. Of those 36 IPRs only 13 were successful in cancelling at least some claims. More specifically, the PTAB cancelled claims in four patents: U.S. Patent Nos. 6,407,213; 7,807,799; 7,846,441; and 7,892,549.
The ’213 patent covers a humanized antibody, while the ’799 patent covers a method of purifying a protein that comprises a CH2/CH3 region by subjecting a composition to affinity chromatography at a specified temperature. The ’441 patent is directed to a method for treating a human patient with a malignant progressing tumor or cancer by administering an antibody and a taxoid, while the ’549 patent covers a method for treatment of a patient with breast cancer that overexpresses ErbB2 receptor comprising administering a combination of an antibody, a taxoid, and a growth inhibitory agent.
Interestingly, the PTAB granted a rare request for rehearing after denying institution of Hospira’s (now Pfizer) petition challenging the ’441 patent.19 The challenged claims are directed to a method for the treatment of a human patient with a malignant progressing tumor or cancer comprising administering a combination of an anti-ErbB2 antibody and a taxoid, “in the absence of an anthracycline derivative.”20 Neither party construed the term “in the absence of an anthracycline derivative” in the petition or preliminary response. However, in denying institution, the PTAB agreed with Patent Owner’s argument that the evidence of record is “insufficient to suggest that an ordinary artisan would have avoided anthracyclines while pursuing the combination therapy with anti-ErbB2 antibody and a taxoid in a treatment regimen.” Hospira requested rehearing, arguing that the PTAB erred in construing the limitation “in the absence of an anthracycline derivative” as requiring “avoidance” of that derivative.21 By construing this term in that way, Hospira contended that Genentech would be able to capture compositions that simply do not include such a derivative.22 Hospira argued that because of this improper construction, the PTAB erred in denying institution of the obviousness ground that included a reference that suggested a therapeutic composition consisting of an anti-ErbB2 antibody and paclitaxel (taxoid) and does not suggest that doxorubicin (an anthracycline derivative) must necessarily be included as part of the same treatment regimen. The PTAB found Hospira’s reasoning persuasive and instituted trial on the previously denied obviousness ground, subsequently cancelling all challenged claims based on that same ground.23
Genentech has asserted patents related to Herceptin in six BPCIA litigations. Five of the six cases settled and Celltrion voluntarily dismissed the sixth on appeal.
C. Bevacizumab (AVASTIN)
In September 2016, Hospira, Inc. filed IPRs against two patents covering Genentech’s Avastin, which is used to treat colorectal, lung, glioblastoma, kidney, cervical, and ovarian cancer. U.S. Patent No. 7,807,799 is directed to methods of purifying antibodies, including claims to purifying anti-VEGF antibodies, while 7,622,115 is directed to methods of treating cancer using bevacizumab.24
The PTAB held that the challenged claims of the ’799 patent were unpatentable as anticipated and obvious over the prior art,25 and the ’115 patent claims unpatentable as obvious.26 The Federal Circuit affirmed in both cases.27, 28
Genentech has filed six complaints against biosimilar developers of AVASTIN. Of the six BPCIA litigations, five have been dismissed and one has settled.
D. Botulinum Toxin Galderma, SA et al v. Medy-Tox Inc. PTAB-PGR2019-00062
In September 2019, Galderma filed a petition for post-grant review of Medy-Tox’s U.S. Patent No. 10,143,728, covering an animal protein-free botulinum composition that exhibits a longer lasting effect than a comparative animal protein containing botulinum composition.29 Botulinum toxin can be used to treat muscle disorders, excessive sweating, and migraine and is commonly used for cosmetic purposes, such as wrinkle reduction.
The petition challenged the ’728 patent on numerous grounds: enablement, indefiniteness, and written description.30 Following institution, Medy-Tox did not file a Response, but instead filed a non-contingent Motion to Amend, seeking to cancel claims 5-6 and replace the remaining claims with substitute claims 11-18, in accordance with the PTAB’s 2019 Pilot Program Concerning Motion to Amend Practice and Procedures.31 The PTAB issued preliminary guidance that Medy-Tox had not satisfied the statutory and regulatory requirements associated with filing a motion to amend in a post-grant review and that Petitioner had shown a reasonable likelihood that proposed substitute claims are unpatentable. In view of the guidance from the PTAB, Medy-Tox then filed a revised non-contingent motion to amend proposing narrower claims by canceling original claim 6 and replacing the remaining claims 1-5 and 7-10 with substitute claims 19-27.32 The PTAB addressed the patentability of proposed substitute claims 19-27 and issued a final written decision on July 16, 2021 and found the substitute claims unpatentable for lack of written description support and lack of enablement.33
As such, patent owners should be aware of the risks involved in amending claims challenged in a petition before considering such a motion. Although the Pilot Program provides an opportunity for the patent owner to get a preview of the PTAB’s thinking on the patentability of amended claims, it is no guarantee of success. In fact, as shown above, following the PTAB’s guidance in its decision on certain amended claims and then filing a second non-contingent motion to amend may bring about additional challenges from the petitioner. In fact, patent owners should be aware that even in situations where the PTAB finds that substitute claims do not enlarge the scope of the original claims, the PTAB can still revisit its preliminary decision and find claims unpatentable. The PTAB makes it very clear that a Preliminary Guidance only provides information indicating initial, preliminary, non-binding views on whether the patent owner has shown a reasonable likelihood that it has satisfied the statutory and regulatory requirements associated with filing a motion to amend and whether petitioner has established a reasonable likelihood that the substitute claims are unpatentable. As such, patent owners should consider other potential avenues for amending claims, such as continuing prosecution, or reissue or reexamination proceedings. And if a patent owner amends claims in an IPR or PGR, the patent owner should carefully consider the possibility that the petitioner may challenge the amended claims for lack of enablement or lack of written description.
E. Adalimumab (HUMIRA): Fresenius Kabi USA, LLC et al v. Coherus Biosciences, Inc. PTAB-PGR2019-0006434
In September 2019, Fresenius Kabi filed a petition challenging U.S. Patent No. 10,155,039, owned by Coherus BioSciences and generally directed to “stable” adalimumab antibody formulations suitable for long-term storage without substantial loss in efficacy. Fresenius asserted that the challenged claims lacked enablement and written description, and were indefinite.35 On March 19, 2020, the PTAB denied institution of the PGR on the ground that Fresenius incorrectly construed the term “stable” in the context of the claims.36 Fresenius construed the term “stable” to refer to long term storage of adalimumab, while Coherus construed “stable” to only require stability suitable for its intended pharmaceutical application. The PTAB agreed with Coherus’ construction. Based on that construction, the PTAB found that Fresenius had failed to demonstrate that the challenged claims are unpatentable for lack of written description because the specification clearly teaches structural features required for achieving a stable adalimumab composition.37 Similarly, the PTAB held that the claims did not need to be enabled for maximum stability as Fresenius had asserted, and thus were enabled.38
Fresenius also argued that claims directed to a composition free of “citrate and phosphate buffers” were indefinite. Fresenius argued that the term was subject to two reasonable constructions: (1) a construction that the claims exclude citrate buffer, phosphate buffer, and the combination of the two or (2) the claims exclude only the combination.39,40 The PTAB held that the term was not indefinite and that the intrinsic evidence taught that the claims must exclude the combination of both buffers. Accordingly, the PTAB held that Fresenius had failed to show indefiniteness.
Petitioners and patent owners alike should avoid construing claims without adequate support in the specification. The case above demonstrates that the outcome can hinge almost entirely on claim construction, especially when the petitioner has construed the claims beyond what the inventor had described as the invention and based its arguments solely on that construction.
The ’039 patent was also involved in a concurrent litigation between Amgen and Coherus in the District of Delaware, which ended up settling on November 26, 2019.41
F. Pegfilgrastim (NEULASTA) Pfizer Inc. et al v. Amgen Inc. PTAB-IPR2021-00528
In February 2021, Pfizer filed a petition challenging a subset of claims in Amgen’s U.S. Patent No. 8,273,707, which covers methods for purifying a protein, such as pegfilgrastim.42 Neulasta decreases the incidence of infection‚ as manifested by febrile neutropenia‚ in patients with nonmyeloid malignancies receiving myelosuppressive anti‐cancer drugs associated with a clinically significant incidence of febrile neutropenia.
The petition raised both anticipation and obviousness grounds. The PTAB then granted institution with respect to all grounds set forth in the petition on August 17, 2021.43 Hospira challenged the claims of the ’707 patent based upon five grounds: two anticipation grounds and three obviousness grounds. The PTAB first considered one of the obviousness grounds and determined that Hospira had established a reasonable likelihood of success at establishing that the challenged claims were obvious. As a result, the PTAB instituted trial on all grounds—in view of SAS Inst., Inc. v. Iancu—without comment on the remaining grounds, instead inviting the parties to develop the record on those grounds at trial. A final written decision is expected by August 17, 2022.
Amgen has sued Mylan, Coherus, Adello Biologics, and Hospira alleging infringement of the ’707 patent.44 Coherus won, and the Federal Circuit affirmed, on the grounds that prosecution history estoppel bars Amgen from succeeding on its infringement claim under the doctrine of equivalents.45 Mylan and Amgen stipulated that the Court could enter a judgment of noninfringement in favor of Mylan. Amgen dismissed all claims against Adello Biologics on November 22, 2019. Hospira filed a motion for summary judgment of noninfringement on August 6, 2021; the parties are waiting for an oral hearing on that motion.
G. Filgrastim (NEUPOGEN) Lupin Limited et al v. Amgen Inc. PTAB-IPR2021-00326
In December 2020, Lupin filed a petition challenging claims in U.S. Patent No. 9,856,287, covering Amgen’s NEUPOGEN (filgrastim).46 Like Neulasta, Neupogen is a drug that treats cancer patients by stimulating growth of white blood cells, making patients less vulnerable to infections.
The ’287 patent covers methods of refolding complex proteins, such as filgrastim, at high concentrations. The PTAB denied institution for multiple reasons.47 The PTAB noted several times in its Institution Decision that Lupin’s expert did not adequately support his conclusions.48 For example, the PTAB agreed with Amgen that a key reference, Vallejo, did not disclose the limitation “wherein the thiol-pair ratio is in the range of 0.001-100.” Lupin’s expert argued that Vallejo’s disclosure taught a thiol-pair ratio of 3, which is within the range claimed. However, the PTAB found that Lupin’s expert did not adequately consider another disclosure in Vallejo which would have impacted the thiol-pair calculation that Lupin’s expert performed. The PTAB also disagreed with Lupin’s argument relating to a limitation calling for the thiol-pair buffer to “maintain the solubility of the preparation,” which the PTAB interpreted as relying on inherency—a notoriously difficult theory to prove at the PTAB.49
Importantly, this decision serves as a reminder that even if a Petitioner does not explicitly rely on inherency for a particular argument, the PTAB may interpret an expert’s testimony as based on inherency if inadequate support is provided for his or her conclusions. Accordingly, Petitioners should take note of this decision and carefully support all conclusions in an expert’s declaration.
Amgen has asserted the ’287 patent against Tanvex BioPharma USA, Accord BioPharma, and Adello Biologics. Amgen dismissed the actions against all three parties in late 2019.50
Notably, despite no assertion of infringement, both Fresenius Kabi USA and Adello Biologics LLC challenged the ’287 patent in post-grant proceedings. Fresenius filed two petitions in 2019. The PTAB denied the first petition and the parties settled the second.51 Adello Biologics filed one petition challenging the ’287 patent, which also settled.52
H. Insulin Glargine (LANTUS) Mylan Pharmaceuticals Inc. v. Sanofi-Aventis Deutschland GmbH PTAB-IPR2019-01657 and IPR2019-01658
In October 2019, Mylan filed two petitions challenging RE47,614 from Sanofi-Aventis covering Lantus (insulin glargine), licensed to treat diabetes. The ’614 patent is directed to a drug delivery device which can house a liquid medicament, such as insulin. 53
In both petitions, Mylan presented a single obviousness ground. Because both obviousness grounds were very similar, the PTAB instituted review of the first petition and denied institution of the second on grounds that the second petition did not contain sufficiently material differences to support instituting an additional IPR of the ’614 patent.54,55
Interestingly, similar to Galderma, SA et al v. Medy-Tox Inc. (PGR2019-00062), Sanofi filed a contingent motion to amend cancelling the challenged claims and substituting new claims 19-22. After Sanofi filed the motion to amend, the PTAB issued Preliminary Guidance determining that Sanofi had shown a reasonable likelihood that it had satisfied the statutory and regulatory requirements associated with filing a motion to amend claims 19, 20, and 22, but not for claim 21, and that Mylan had shown a reasonable likelihood that claims 19, 20, and 22 are unpatentable, but not claim 21.56 Sanofi subsequently withdrew the motion to amend. As a result, the PTAB only needed to consider the instituted claims, which the PTAB subsequently cancelled in view of the prior art.57
CONCLUSION
Very few petitions challenging biologics patents go through to a final written decision, making distinct trends specific to large molecules difficult to identify. Instead, the challenges seem to rise and fall on issues that arise in post-grant proceedings regardless of technology: claim construction, failure of proof, public accessibility to prior art, etc. In particular, decisions by the PTAB recently have suggested that patent owners of biologics patents should think carefully before amending claims even after receiving preliminary guidance from the PTAB. In addition, given the PTAB’s willingness to deny institution purely on claim construction grounds, practitioners should pay special attention to claim construction positions to make sure they are adequately supported. That said, at least 23 petitions challenging biologic patents have been filed this year alone, which will provide more decisions to guide practitioners in this field.
1. PTAB Orange Book patent/biologic patent study: FY21 Q3 (June 2021) Update, https://www.uspto.gov/sites/default/files/documents/PTABOB-biologicpatentstudy8.10.2021draftupdatedthruJune2021.pdf, 2021.
2.Id. at 2.
3. Id. at 5.
4. Id. at 5-6.
5.Id. at 11-12.
6. Id. at 10.
7. Id. at 11.
8. Id. at 12.
9. Id.
10. Id. at 13
11. Id. at 14.
12. Id. at 17-18.
13. Pfizer Inc. v. Biogen Inc., IPR2017-01168, Paper 59 (P.T.A.B. Oct. 31, 2018).
14. Id.
15. Celltrion Inc. v. Biogen Inc., IPR2017-01095, Paper 2 (P.T.A.B. March 15, 2017).
16.Celltrion IPR2017-01095, , Paper 60 at 70-71(P.T.A.B. Oct. 4, 2018).
17.Celltrion Inc. v. Genentech Inc., IPR2016-01614, Paper 2 (P.T.A.B. Aug. 15, 2016); Pfizer Inc. v. Genentech Inc., IPR2017-01115, Paper 2 (P.T.A.B. Mar. 24, 2017).
18. Celltrion IPR2016-01614, Paper 12 at 8-9 (P.T.A.B., Feb. 24, 2017).
19. Pfizer Inc. v. Genentech Inc., IPR2017-00731, Paper 29 (P.T.A.B. Jan. 20, 2017).
20. Id. at 17.
21. Id.
22. Id. at 17-18.
23.Pfizer Inc., IPR 2017-00731, Paper 120 at 44 (P.T.A.B. Oct. 3, 2018).
24.Hospira, Inc. et al v. Genentech, Inc., IPR2016-01837, Paper 1 (P.T.A.B. Sept. 16, 2016); Hospira, Inc. et al v. Genentech, Inc., IPR2016-01771, Paper 1 (P.T.A.B. Sept. 9, 2016).
25.Hospira, Inc. et al v. Genentech, Inc., IPR2016-01837, Paper 1.
26.Hospira, Inc. et al v. Genentech, Inc., IPR2016-01771, Paper 40.
27.Genentech v. Hospira & U.S., Case No. 18-1933 (Fed. Cir. 2020).
28.Genentech v. Hospira & U.S., Case No. 18-1959 (Fed. Cir. 2019).
29. Galderma, S.A. v. Medy-Tox Inc., PGR2019-00062, Paper 2 (P.T.A.B. Sept. 4, 2019).
30.Id. at 11.
31.Galderma, S.A. v. Medy-Tox Inc., PGR2019-00062, Paper 21 (P.T.A.B. Aug. 5, 2020).
32.Galderma, S.A., PGR2019-0062, ., Paper 30 (P.T.A.B. Dec. 11, 2020).
33.Galderma, S.A., PGR2019-0062Paper 66 at 33-34 (P.T.A.B. July 16, 2021).
34. Fresenius submitted a Request for Rehearing, which the Board denied on January 27, 2021.
35.Fresenius Kabi USA, LLC v. Coherus Biosciences, Inc., PGR2019-00064, Paper 3 (P.T.A.B. Sept. 17, 2019).
36.Fresenius Kabi USA, PGR2019-0064. Paper 10 at 11-18 (P.T.A.B. Sept. 17, 2019).
37. Id. at 14-15.
38. Id. at 15-18.
39. Id. at 18-19.
40. Fresenius Kabi USA, PGR2019-0064, Paper 12 (P.T.A.B. Jan, 27, 2021).
41. Coherus Biosciences, Inc. v. Amgen Inc., Case No. 1:19-cv-00139 (DDE).
42. Pfizer Inc. v. Amgen Inc., IPR2021-00528, Paper 2 (P.T.A.B. Feb. 10, 2021).
43. Pfizer, IPR2021-00528, Paper 7 (P.T.A.B. Aug. 17, 2021).
44.Amgen Inc. et al. v. Coherus Biosciences, Inc., Case No. 1:17-cv-00546 (DDE); Amgen Inc. et al. v. Adello Biologics, LLC, Case No. 2:18-cv-03347 (DNJ); Amgen Inc. et al. v. Hospira, Inc. et al., Case No. 1:20-cv-00201 (DDE); Amgen Inc. et al. v. Mylan Inc. et al., Case No. 2:17-cv-01235 (WDPA).
45. Amgen Inc. v. Coherus Biosciences, Inc., Case No. 2018-1993 (Fed. Cir. 2019).
46.Lupin Limited v. Amgen Inc., IPR2021-00326, Paper 1 (P.T.A.B. Dec. 15, 2020).
47. Lupin, IPR2021-00326, Paper 8 (P.T.A.B. July 12, 2021).
48.Id. at 8-12.
49. Id.
50.Amgen Inc. et al. v. Tanvex BioPharma USA, Inc. et al., Case No. 3:19-cv- 01374 (SDCA); Amgen Inc. et al v. Accord BioPharma, Case No. 0:18-cv-61828 (SDFL); Amgen Inc. et al. v. Adello Biologics, LLC, Case No. 2:18-cv-03347 (DNJ).
51.Fresenius Kabi USA, LLC et al. v. Amgen, Inc. et al., IPR2019-00971, Paper 13 (P.T.A.B. Oct. 16, 2019); Fresenius Kabi USA, LLC et al. v. Amgen, Inc. et al., IPR2020-00314, Paper 17 (P.T.A.B. June 19, 2020).
52. See Adello Biologics, LLC et al. v. Amgen Inc. et al., PGR2019-00001, Paper 28 (P.T.A.B. Dec. 6, 2019).
53. Mylan Pharmaceuticals Inc. v. Sanofi-Aventis Deutschland GmbH, IPR2019-01657, Paper 3 (P.T.A.B. Oct. 7, 2019); Mylan Pharmaceuticals Inc. v. Sanofi-Aventis Deutschland GmbH, IPR2019-01658, Paper 3 (P.T.A.B. Oct. 7, 2019).
54.Mylan Pharmaceuticals Inc. v. Sanofi-Aventis Deutschland GmbH, IPR2019-01657, Paper 9 (P.T.A.B. Apr. 2, 2020); Mylan Pharmaceuticals Inc. v. Sanofi-Aventis Deutschland GmbH, IPR2019-01658, Paper 9 (P.T.A.B. Apr. 2, 2020).
55.Id.
56.Mylan Pharmaceuticals Inc. v. Sanofi-Aventis Deutschland GmbH, IPR2019-01657, Paper 27, 3-4, 8 (P.T.A.B. Oct. 14, 2020).
57.Mylan, Paper 39 at 58-59.
This article appeared in the 2021 PTAB Year in Review: Analysis & Trends report. To view our graphs on Data and Trends, please click here.
Related Industries

Receive insights from the most respected practitioners of IP law, straight to your inbox.
Subscribe for Updates